In one of my previous posts, I talked about immunostaining and touched on the principles and procedures of Western blotting, a technique that is widely used to quantify protein expression. Now, I’m going to shift gears and talk about another powerful molecular tool that is often used in research labs: zymography. Distinct from Western blotting, this technique allows for the visualization and quantification of enzymatic activity, making it a valuable method for investigating various biological processes.
A Very Brief History
Zymography has its roots in the early days of biochemistry and protein analysis. The first reports of zymography-like techniques can be traced back to the 1920s, when scientists were studying the activity of digestive enzymes in animals.
The modern zymography technique was first described in 1980 by Heussen and Dowdle, who used gelatin-containing polyacrylamide gels to study the activity of matrix metalloproteinases (MMPs) in biological samples1. This was a significant development in the field, because it allowed researchers to directly visualize the activity of these enzymes without the need for extensive purification.
Zymography vs. Western Blot
When I first heard about zymography in my lab, I must admit that I confused it with Western blotting. After all, both techniques involve the separation through electrophoresis and detection of proteins on a gel. However, I was far from being right, because there are some important differences between the two methods.
As mentioned in the intro paragraph, the most crucial difference between Western blot and zymography lies in what they’re used to measure. Western blotting is primarily used to quantify protein expression levels, while zymography measures enzymatic activity.
In Western blotting, proteins are first separated based on their size using polyacrylamide gels and then transferred onto a solid membrane. In contrast, zymography involves incorporating a substrate specific to the enzyme of interest directly into the polyacrylamide gel, so there is no need for a membrane. After electrophoresis, the gel is incubated under conditions that allow the enzyme to interact with the substrate, resulting in clear zones of lysis where the enzyme has been active.
Common Applications
Zymography is commonly used to study the activity of Matrix Metalloproteinases (MMPs). MMPs play crucial roles in numerous biological processes. However, dysregulation of MMP activity has also been implicated in several pathological conditions, including cancer, arthritis, and cardiovascular diseases2.
In cancer cells, MMPs are often overexpressed and contribute to tumor invasion by breaking down extracellular matrix (ECM) proteins, proteins that provide structural support to cells and play a crucial role in cell signaling, proliferation, and differentiation. The breakdown of ECM by MMPs enables cancer cells to migrate to other tissues3.
In my lab, we look at MMP activity to study ischemia-reperfusion (IR) injury. During IR injury, the tissue experiences reduced blood supply (ischemia) followed by the restoration of blood flow (reperfusion). The sudden reintroduction of oxygen and nutrients can cause tissue damage due to the formation of free radicals, reactive oxygen species (ROS) and inflammation. MMP-induced degradation of ECM proteins can promote inflammation, which can worsen tissue damage.
In both cases, zymography can be used to quantify MMP activity. By doing so, researchers can better understand the role of MMP activity in cancer progression, IR injury, or other studies, and look for potential targets for therapy. In clinical research, researchers can use zymography to evaluate the activity of inhibitors that may reduce the activity of MMPs. This can help identify promising drugs for further study and potentially lead to the development of new treatments for diseases or conditions.
A Very Simple Protocol
- Prepare the gel: Pour a polyacrylamide gel with a gelatin substrate. This substrate will be cleaved by the enzyme of interest, leaving a clear band on the gel where the substrate was degraded.
- Add the sample: Mix your sample with a sample buffer, and add it to the gel wells.
- Electrophoresis: Run the gel under an electric field to separate the proteins.
- Renaturation: Soak the gel in a renaturation buffer to refold the enzymes that have been denatured by the electrophoresis process.
- Substrate incubation: Incubate the gel in a substrate buffer containing a substrate specific to the enzyme of interest. The substrate will bind to and be cleaved by the enzyme, leaving a clear band on the gel.
- Staining: Stain the gel with Coomassie Blue or a silver stain to visualize the bands.
- Analysis: Analyze the results to determine the activity of the enzyme of interest in the sample.
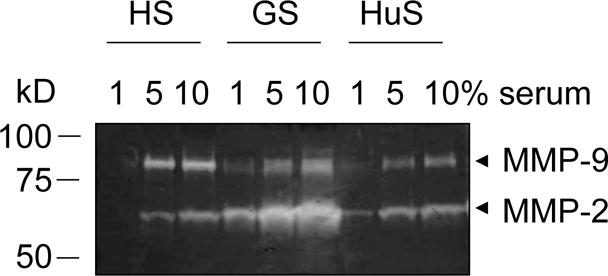
Final Thoughts
Zymography is an important technique that lets researchers measure the activity of enzymes like MMPs. With zymography, we can study the important roles these enzymes play in different biological processes and diseases like cancer and IR injury. Right now, I’m in the process of learning how to run zymography in my own lab, so I’m hoping to get some sweet results soon.
References
- Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb; 102(1):196-202. doi: 10.1016/0003-2697(80)90338-3. PMID: 7188842.
- Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999 Jul 30;274(31):21491-4. doi: 10.1074/jbc.274.31.21491. PMID: 10419448.
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67-8. doi: 10.1038/284067a0. PMID: 6243750.
- Tajhya RB, Patel RS, Beeton C. Detection of Matrix Metalloproteinases by Zymography. Methods Mol Biol. 2017;1579:231-244. doi: 10.1007/978-1-4939-6863-3_12. PMID: 28299740; PMCID: PMC5465868.
Leave a Reply